
White Papers
White Papers
Are you ready for the next UDI deadline? - Datalogic - Datalogic

Learn about the new
Unique Device Identification
Regulations for medical devices
and how to ensure compliance.
Complete traceability of medical instruments, implants, devices and consumables means greater safety for the patient.
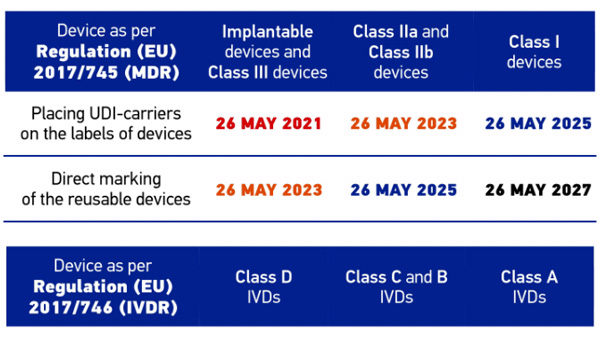
A relevant step in UDI implementation in Europe is mandatory UDI-carrier placement on implantable and class III devices starting May 2021 in accordance with Regulation (EU) 2017/745 (MDR - Medical Device Regulation). Reliable identification and data acquisition systems are an important basis to allow manufacturers, distributors and users to meet the new requirements.
This white paper gives you an overview of the UDI regulations and the various technical solutions for marking and automatic identification of medical devices.
 Learn how to comply easily with UDI marking requirements on medical devices bringing benefits to all involved - including:
Learn how to comply easily with UDI marking requirements on medical devices bringing benefits to all involved - including:
- Clearly labelled medical devices are easier to trace – from the user to the manufacturer
- Quick and easy identification and location of the involved products in case of incident
- Easier identification of illegally marketed medical devices
and many others.

Download your complimentary White Paper by filling out the form 


